
Experience Marvin 3.0
Discover the new Marvin – and manage the complete lifecycle of a clinical trial in one single integrated solution.
Marvin is the state-of-the-art EDC solution for academics.
Get in touch with our team of experts
Fill out the form below and our experts will contact you.
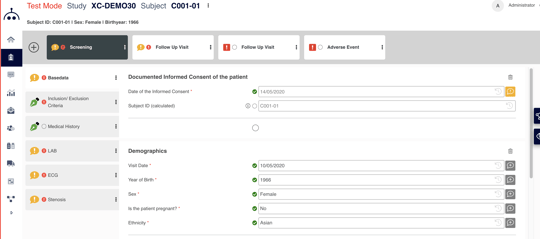
MARVIN 3.0
DESIGNED FROM THE GROUND UP WITH ITS END USERS’ NEEDS IN MIND.
With Marvin clinical trials become easy and cost-effective, especially for academic institutions.
FLEXIBLE FOR ANY TYPE OF STUDY
Easy Study Set-up and Management
- Save time and money
- Simplicity combined with complex, customizable work flows
- Seamless data flow
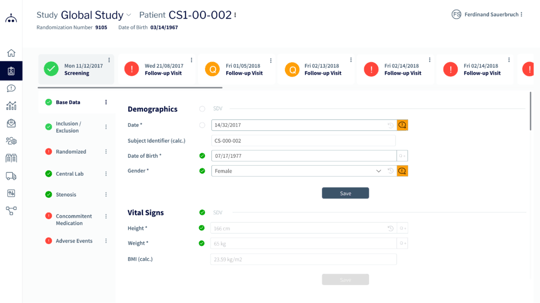
BUILT FOR GLOBAL BUSINESS
Impressive Universal Capabilities
- Multi-language
- Integrated IWRS and multi trial capabilitites
- Single sign on
STRONG INTERFACING
Beyond Data Capture
- Quick and easy implementation of mid study changes
- Advanced query management
- Flexible for any type of study
- Complete Audit Trail
- (S)AE Notifications
- Electronic Signatures
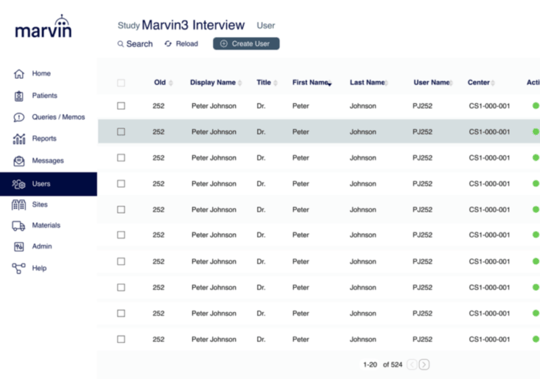
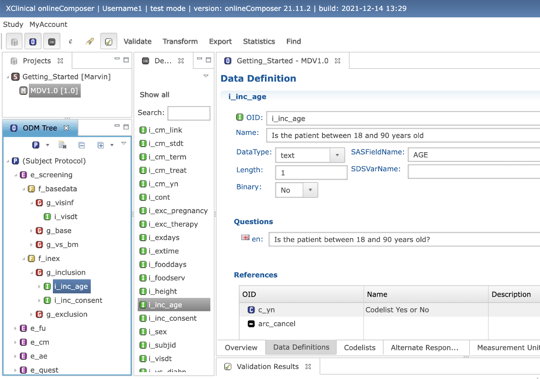
eCRF DESIGN
Composer
- Smart metadata library functionality
- Easy setup and management of amendments
- Automatic output of the annoted CRF, data validation plan and more
- Ensures compliance with the international CDISC ODM standard
Why choose Marvin?
"90% of our customers chose Marvin either for its usability or quick set-up."

Rupert Sedlmayr
Quality Manager, EvidentIQ