Marvin edc - simplify complex studies
Marvin EDC by EvidentIQ is the solution to support you with your complex Clinical Trial. Marvin is a reliable EDC system developed in Germany and trusted by research sites since 2002. We have supported CROs and sponsors in complex studies for over 20 years.

EvidentIQ StudyMetrIQs for more insights in your study data.
Request more information
Fill out the form below and download more information.
Marvin EDC – Trusted by Sites. Built in Germany.
For over two decades, we’ve been a trusted partner to CROs and sponsors, supporting them through complex clinical studies with reliability and expertise. Our responsive, bilingual team—fluent in both English and German—understands the real-world challenges our clients face and is ready to help at every step. As one of the early adopters of CDISC certification, compliance has always been more than a requirement for us—it’s part of our DNA. And because we design with clinical sites in mind, our solutions are intuitive and easy to use, helping reduce data-entry errors and ensuring smooth adoption across study teams.
Clients often tell us they feel “understood” when working with us. That’s not a coincidence—our support team does not just fix problems, they help you move faster and with more confidence.
How Marvin supports your Complex Clinical Trial
1. EDC with an eCRF data-tree structure
Marvin organizes all data collected during your trial in a hierarchical, tree-based structure. That’s not just a design choice—it’s a strategic advantage:
- Clarity: Each patient’s data is structured clearly—parent nodes (e.g. Patient) link to child nodes (e.g. Medical History, Labs).
- Navigation: Jumping between sections is fast and intuitive.
- Validation: Built-in rules ensure only complete and consistent data is submitted.
- Standardization: Aligned with clinical coding systems like WHODrug or MedDRA.
- Flexibility: Supports different data types—text, numbers, dates, categories—all in one place.
This structure empowers your teams to review, monitor, and analyze trial data with ease—across all sites and all phases.
It’s one of the reasons Marvin scales so well for complex, multi-center studies.
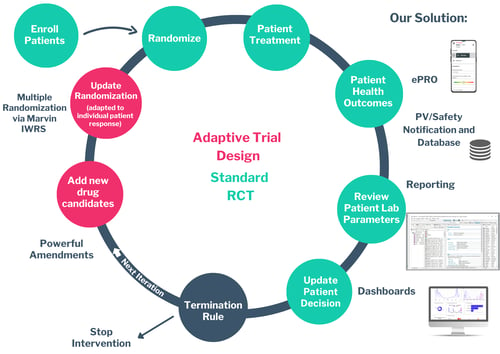
2. Marvin the specialist for complex studies
Whether you're running a traditional RCT, a decentralized trial, or something more advanced like an adaptive design, Marvin adapts to your needs. Our platform is engineered to accommodate any workflow complexity—without compromising on performance or compliance.
From screening to randomization, dosing, and follow-up, Marvin lets you configure your trial logic exactly as you need it. Even adaptive trial designs—with interim analyses, cohort adjustments, or dose escalations—can be fully implemented and executed within Marvin.
And the best part? We make it look easy.
What Our Customers Are Saying...
"I can use Marvin for all study designs I can think of, no matter the complexity."
Clinical Data Manager
Global Pharma
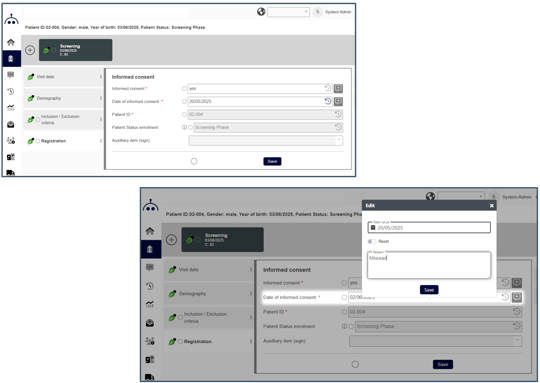
3. Site-friendly design
Even with all that complexity - Marvin stays easy to use. What makes Marvin truly user-friendly?
- Clean, role-specific dashboards – no distractions
- Smart form logic – fewer clicks, fewer errors
- Guided workflows – no need to figure it out
- Built for real users – not just data managers
Marvin is the right choice to simplify your Clinical Trial

-
Only EDC with an eCRF data tree structure based on CDISC ODM Data Model
-
Trusted partner
-
Manage any workflow of your Clinical Study
-
Marvin is user-friendly
Get more Information about Marvin EDC now.
Ensuring Regulatory Compliance and Industry Standards with Confidence






EvidentIQ is a leading provider of modern eClinical solutions for clinical research experts. The CDISC-certified EDC software Marvin is perfect for CROs and academic institutions and includes modules for randomization, ePRO, Trial Supply Management, CDISC tabulation, reporting, coding and more.